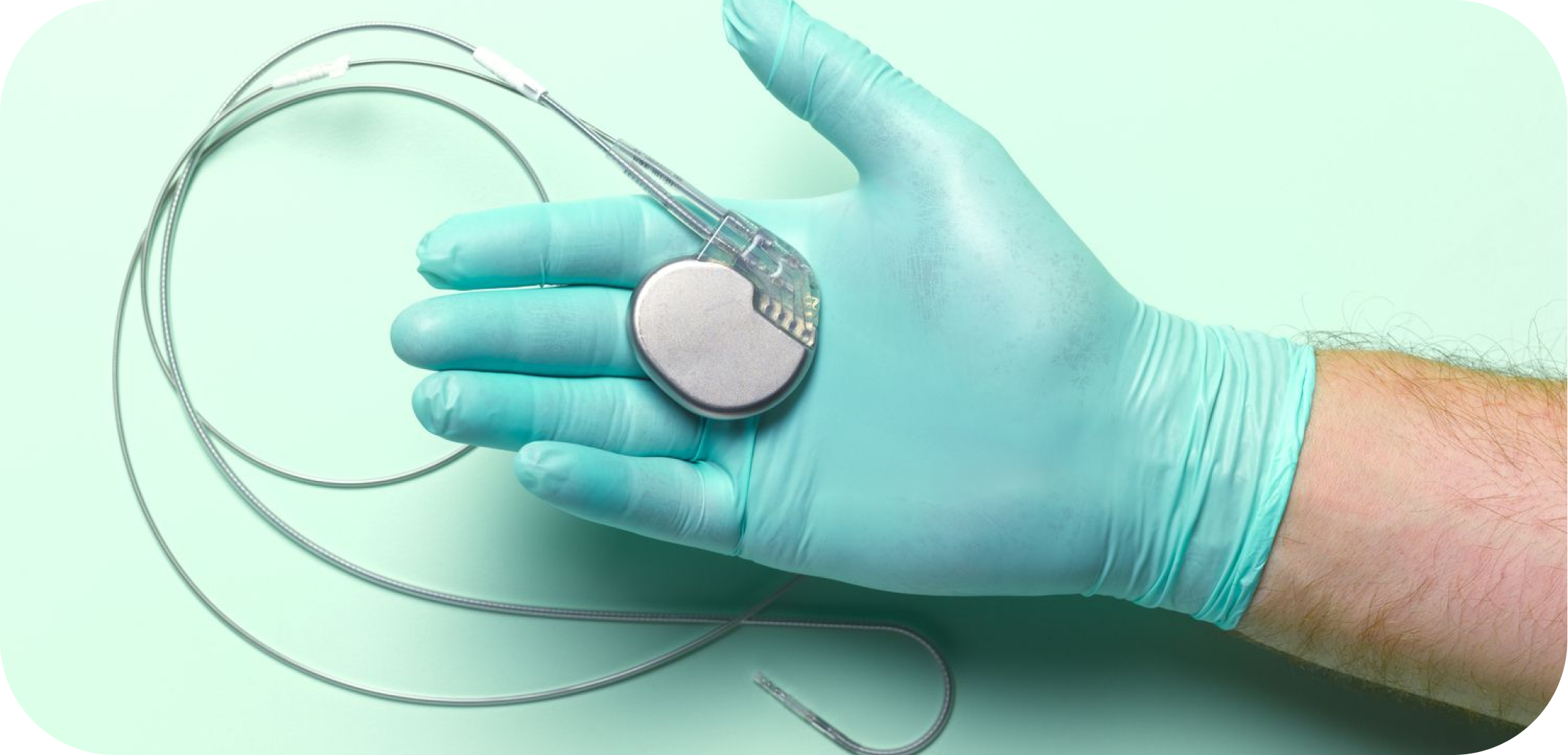
Solutions for Medical Devices with Integrated Electronics
#MEDISTRI
#MEDISTRI
Medistri continues to be at the forefront of regulatory requirements and quality proficiency.As the medical industry advances, devices are becoming more compact & connected. Many of today’s innovations include integrated electronics - microchips, sensors, batteries, or embedded software, that allow for monitoring, adjustment, or data transmission. These features improve patient outcomes, but they also create new challenges when it comes to sterilisation.
At Medistri, we work with manufacturers to identify and validate sterilisation methods that protect both patient safety and product performance. For medical devices with integrated electronics, this balance is essential.
Medistri’s Sterilisation Factors for Integrated Electronics
Medical devices with integrated electronics, often classified as active devices, rely on electrical components to perform their function. This includes pacemakers, insulin pumps, wearable monitors, implantable neurostimulators, and advanced surgical tools. These devices may include sensitive components that cannot tolerate the high heat, humidity, or pressure used in traditional sterilisation methods.
The choice of sterilisation process must be guided by several factors: the temperature sensitivity of the materials, the device’s resistance to moisture, the complexity of its design, and its regulatory classification. Most importantly, the method must not compromise the function or reliability of the electronics inside.
Understanding the Limitations of Steam for Sensitive Devices
Steam sterilisation - defined by ISO 17665 - is commonly used in hospitals and manufacturing. It is effective, but also aggressive. The process typically exposes devices to temperatures ranging from 121°C to 134°C under high pressure and saturated steam.
For devices with electronics, this environment poses risks. Prolonged heat can distort plastic housings, degrade adhesives, or shorten battery lifespan. Moisture may cause corrosion, disrupt calibration, or interfere with circuitry. Even well-sealed components may not be fully protected against these effects. Unless a device is specifically engineered to endure this process, steam sterilisation is often not suitable.
EO Sterilisation for Medical Devices with Integrated Electronics
Ethylene oxide (EO) sterilisation offers a low-temperature, low-moisture alternative. Operating between 30°C and 60°C, EO is compatible with a wide range of materials and is especially appropriate for devices that integrate sensitive electronics. It penetrates packaging and internal geometries effectively, making it possible to sterilise fully assembled and packaged products.
Most importantly, EO sterilisation, as defined by ISO 11135, allows manufacturers to achieve the required Sterility Assurance Level while maintaining the integrity of the device’s structure and electronics. When validated correctly, it ensures both safety and performance, without compromising innovation.
Early-Stage Support Development
For manufacturers still in the R&D phase, Medistri provides sterilisation of small batches - helping teams evaluate sterilisation compatibility early in the process. This shortens development cycles and reduces the risk of late-stage surprises. By working together from the start, we help ensure that your device is designed with sterilisation in mind.
End-to-End EO Sterilisation Expertise
Medistri offers complete EO sterilisation support - from early-stage development to full validation and routine processing. Our facility is equipped to manage everything from small prototype batches to high-volume production. We work with you to design sterilisation cycles tailored to your product, ensuring compliance with regulatory expectations.
Our integrated laboratory can conduct all required testing, including bioburden assessments, EO residual analysis, and sterility testing. During the validation process we can also support our customer with functional testing after sterilisation.
Learn more about how Medistri supports the sterilisation of medical devices with integrated electronics. To explore how our EO sterilisation solutions help preserve performance, ensure compliance, and accelerate development, visit our website here or contact our team at contact@medistri.com.
— The Medistri Team
#Medistri
Follow us: Website, Blog, LinkedIn, Facebook, Twitter, Instagram and Medium.